Background: Somatic mutations in isocitrate dehydrogenase 1 (IDH1) are reported in 6-10% of patients (pts) with acute myeloid leukemia (AML), resulting in production of the oncometabolite D-2-hydroxyglutarate. Ivosidenib (IVO) is an oral, potent, targeted inhibitor of mutant IDH1 (mIDH1) and is FDA-approved for the treatment of mIDH1 relapsed or refractory (R/R) AML and mIDH1 newly diagnosed (ND) AML in adults ≥ 75 years of age or with comorbidities precluding intensive induction chemotherapy. In a phase 1 study (NCT02074839), durable remissions in pts with mIDH1 ND AML (n = 33) were achieved with IVO, with a complete remission (CR) plus CR with partial hematologic recovery (CRh) rate of 42.4%, and median overall survival of 12.6 months (mo), as of 02Nov2018. The most frequent co-occurring mutations at baseline were ASXL1, DNMT3A, RUNX1, SRSF2, TET2, and NRAS.
Aim: To characterize the longitudinal evolution of gene mutations in pts with mIDH1 ND AML treated with IVO 500 mg once daily, including relapse mechanisms and depth of molecular response for mIDH1 and co-occurring mutations.
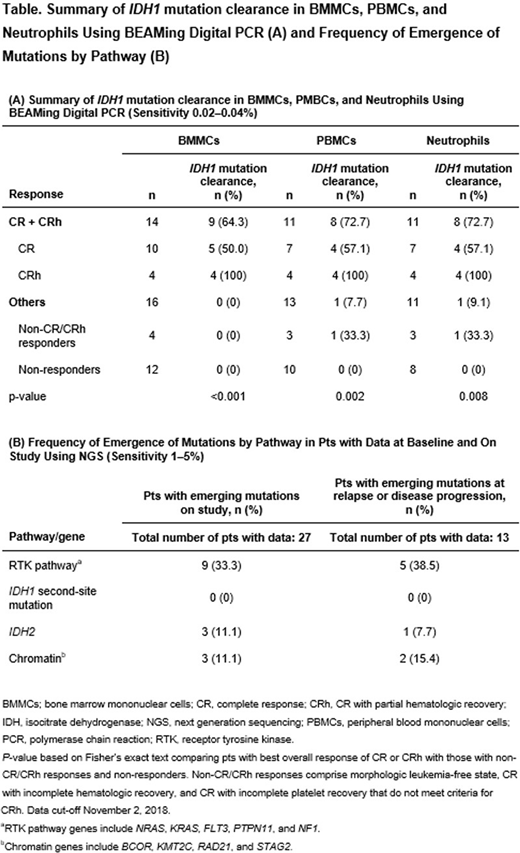
Methods: The mIDH1 variant allele frequency (VAF) was assessed in bone marrow mononuclear cells (BMMCs), peripheral blood mononuclear cells (PBMCs), and neutrophils using BEAMing digital polymerase chain reaction (PCR) technology (Sysmex Inostics, Inc.), which has a lower limit of detection for mIDH1 of 0.02-0.04%. Deep IDH1 mutation clearance (MC) was defined as reduction in mIDH1 VAF to below the limit of detection for ≥ 1 on-treatment timepoint. Baseline and longitudinal co-occurring mutation profiling was conducted on BMMC or PBMC samples by next-generation sequencing (NGS; detection sensitivity of 1-5%). Single-cell targeted DNA sequencing (DNA-seq) was performed on PBMCs using a microfluidic platform (Tapestri®). The clinical data cut-off for this analysis was 02Nov2018.
Results: In pts who achieved a best response of CR or CRh, the IDH1-MC rate in BMMCs was 64.3% (9/14), and 72.7% (8/11) in both PBMCs and neutrophils, by sensitive digital PCR (Table). Median time to IDH1-MC was 7.4 mo (BMMCs), 6.9 mo (PBMCs), and 5.1 mo (neutrophils) in pts achieving CR or CRh. IDH1-MC was significantly associated with a best response of CR or CRh (p < 0.001, Table). Overall survival at 12 mo was 88.9% (95% CI 43.3, 98.4) for pts with IDH1-MC in BMMCs (n = 9), as compared with 38.5% (95% CI 17.7, 59.0) for pts who did not achieve IDH1-MC (n = 21). The longitudinal evolution of mIDH1 and co-occurring mutations during IVO treatment was profiled by NGS in 27 pts. In 13 pts who achieved a best response of CR/CRh and with available data, non-DTA (DNMT3A, TET2, ASXL1) gene mutation clearance was observed for IDH1 (11/13), RUNX1 (2/4), SRSF2 (2/3), and NPM1 (2/2). One pt had all co-occurring mutations (IDH1, FLT3, and NPM1) cleared by IVO monotherapy, and maintained CR for 30.2 mo as of the data cut-off. The most frequent mutations acquired at relapse or disease progression following IVO treatment were mutations in receptor tyrosine kinase (RTK) pathway genes (38.5%; 5/13), followed by mutations in chromatin remodeling (15.4%; 2/13) and IDH2 (7.7%; 1/13; Table). No IDH1 second-site mutations were observed in this cohort by NGS; however, emergence of an IDH1 R119P second-site mutation was observed in 1 pt using a single-cell DNA-seq assay.
Conclusions:IDH1-MC across examined cell types (BMMCs, PBMC, and neutrophils) suggests that IVO can alter the biology of mIDH1 ND AML via reduction of the primitive mIDH1 cells. Similar to previous findings in R/R AML, the observed trend of improved overall survival in pts with deep molecular remission (ie, IDH1-MC) warrants further investigation in a larger pt cohort. Relapse is mediated by diverse emergent mutations, most frequently in RTK pathway genes, chromatin remodeling genes, and IDH2. Clonal architecture and evolution in ~ 20 pts revealed by single-cell DNA-seq analysis will be presented.
Choe:Agios Pharmaceuticals: Current Employment, Current equity holder in private company. Wang:Agios Pharmaceuticals: Current Employment, Current equity holder in private company. Roboz:Array BioPharma: Consultancy; MEI Pharma: Consultancy; Helsinn: Consultancy; Epizyme: Consultancy; Jasper Therapeutics: Consultancy; Cellectis: Research Funding; Trovagene: Consultancy; Takeda: Consultancy; Otsuka: Consultancy; Bayer: Consultancy; Celltrion: Consultancy; Eisai: Consultancy; Jazz: Consultancy; Roche/Genentech: Consultancy; Sandoz: Consultancy; Abbvie: Consultancy; Pfizer: Consultancy; Novartis: Consultancy; Janssen: Consultancy; Celgene: Consultancy; Astex: Consultancy; Amphivena: Consultancy; Agios: Consultancy; Orsenix: Consultancy; AstraZeneca: Consultancy; Daiichi Sankyo: Consultancy; Astellas: Consultancy; Argenx: Consultancy; Actinium: Consultancy. DiNardo:Agios: Consultancy, Honoraria, Research Funding; Jazz: Honoraria; AbbVie: Consultancy, Honoraria, Research Funding; ImmuneOnc: Honoraria; Syros: Honoraria; Daiichi Sankyo: Consultancy, Honoraria, Research Funding; Novartis: Consultancy; Notable Labs: Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria; Calithera: Research Funding; Celgene: Consultancy, Honoraria, Research Funding; MedImmune: Honoraria. Stein:Syros: Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Consultancy; Syndax: Consultancy, Research Funding; Amgen: Consultancy; Abbvie: Consultancy; PTC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Agios Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Biotheryx: Consultancy; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bayer: Research Funding; Daiichi-Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees. Mims:Jazz Pharmaceuticals: Other: Data Safety Monitoring Board; Syndax Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Kura Oncology: Membership on an entity's Board of Directors or advisory committees; Leukemia and Lymphoma Society: Other: Senior Medical Director for Beat AML Study; Novartis: Speakers Bureau; Abbvie: Membership on an entity's Board of Directors or advisory committees; Agios: Consultancy. Watts:Pfizer: Consultancy; Celgene: Consultancy; Jazz: Consultancy, Speakers Bureau; Takeda: Research Funding. Fan:Agios Pharmaceuticals: Current Employment, Current equity holder in private company. Nejad:Agios Pharmaceuticals: Current Employment, Current equity holder in private company. Zhang:Agios Pharmaceuticals: Current Employment, Current equity holder in private company. Liu:Agios Pharmaceuticals: Current Employment, Current equity holder in private company. Attar:Agios Pharmaceuticals: Current Employment, Current equity holder in private company. Wu:Agios Pharmaceuticals: Current Employment, Current equity holder in private company. Stone:Takeda: Consultancy; Arog: Research Funding; Argenx: Consultancy, Other: Data and safety monitoring board; Agios: Consultancy, Research Funding; Actinium: Consultancy; Novartis: Consultancy, Research Funding; Jazz: Consultancy; AbbVie: Consultancy, Research Funding; Gemoab: Consultancy; Elevate: Consultancy; Daiichi-Sankyo: Consultancy; Stemline: Consultancy; Syndax: Consultancy; Syntrix: Consultancy; Hoffman LaRoche: Consultancy; Macrogenics: Consultancy; Janssen: Consultancy; Syros: Consultancy; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Consultancy; Biolinerx: Consultancy; Celgene: Consultancy, Other: Data and safety monitoring board; Trovagene: Consultancy; Pfizer: Consultancy; Otsuka: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal